By combining a Blow-Fill-Seal Container with a pen needle hub, we’re making drug delivery

The Apiject Platform can bring together Blow-Fill-Seal (BFS) containers and attachable delivery systems in an infinite variety of ways. At Studio One – Apiject’s design innovation center in London – a world-class team of device designers and engineers invent ways that BFS-based prefilled drug delivery systems can reliably and affordably deliver medicines that are needed by patients in a wide range of environments.
From intramuscular to intradermal, very small dose to large infusions, standardized to variable dosing, the team at Studio One is designing innovative ways for more drugs to reach more patients in a safe and reliable system.

Over the last 5 years, the Studio One team has produced hundreds of concepts and dozens of prototypes of prefilled injectors, all based on the Platform’s design principles of bringing together BFS containers and needle hubs.
The Studio One team is led by Marc Koska, OBE. Mr. Koska is the inventor of the K1 auto-disable syringe, and has been credited with saving more than 15 million lives by reducing the spread of iatrogenic infections from unsafe syringe use.
Next generation pre-filled injectable device redefining drug delivery and increasing access to those who need it most.*
*All devices and prototypes shown have not been cleared by the FDA or any other regulator.

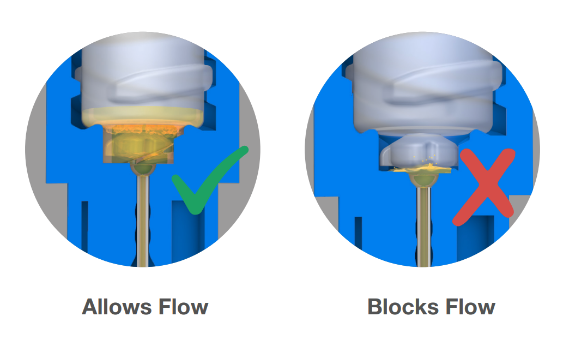
The device features a one-way valve integrated into the soft LDPE container. When the user twists the needle hub, the valve tears, opening the container and allowing liquid to flow into the cannula for dose delivery. This valve, working in tandem with other design elements, ensures the device cannot be reused.
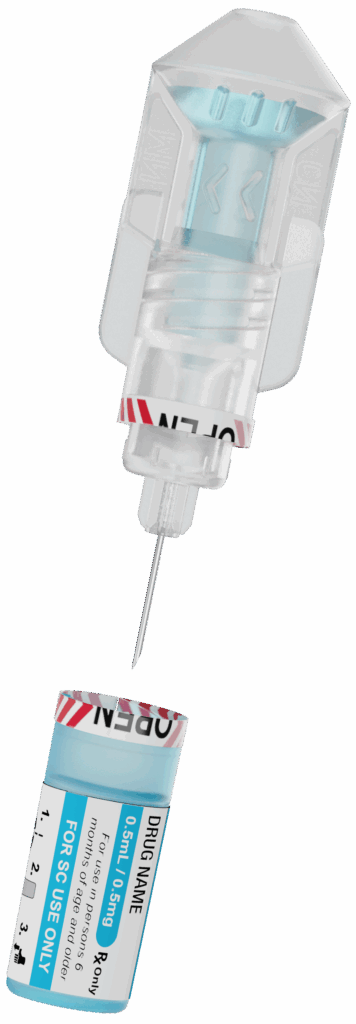






By combining a Blow-Fill-Seal Container with a pen needle hub, we’re making drug delivery
Contact us today to learn more about Apiject and easy first steps to see if our Platform may be right for your sterile liquid product.