
Device
Development

A Prefilled Delivery System for Your Injectables
From simple generics to ultracold vaccines, Apiject is collaborating with a wide range of pharma partners to develop the right prefilled delivery system* for their products to meet market and company goals and deliver results.
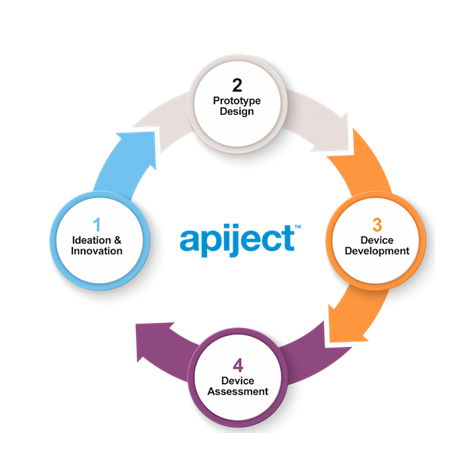
A Trusted Process that Works
Introducing a new platform requires experience and risk mitigation to ensure success. This is why our experts have designed an end-to-end device development process that is customized for your specific drug product, target markets, customers, and economics considerations.
Development
Process
From gathering requirements to prototype testing to feasibility filling, Apiject offers pharma partners an end-to-end development process that will reduce risk and improve outcomes.
Product & Market Requirements
Proof of Concept
Prototype Testing
Mold Design
Feasibility Filling
BFS Training & Partnerships
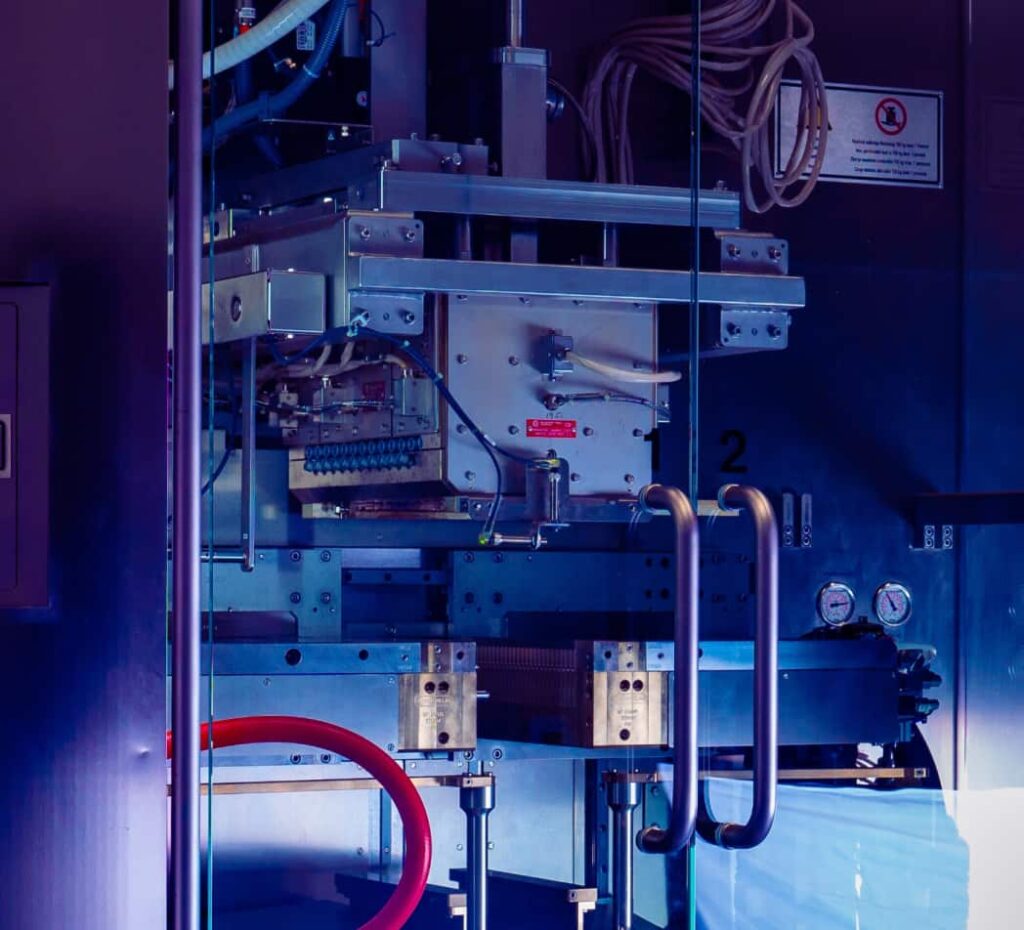
Technology Development Center
With multiple BFS machines, feasibility testing, and a mold design and engineering shop, our 15,000 sq. ft. facility outside of Orlando, FL, allows us to offer our pharma partners comprehensive BFS capabilities to develop the ideal prefilled delivery system for any injectable.

Designing Future Devices for the Apiject Platform
The future of the Apiject Platform is being created by our team of designers and inventors in London. Known as Studio One, the team, led by Marc Koska OBE, is developing and exploring innovative ways to expand the reach of the Platform.
Get in Touch!
Contact us today to learn more about Apiject and easy first steps to see if our Platform may be right for your sterile liquid product.