
ISO
Symbols Glossary
Last Updated: February 3, 2025
The following is a glossary of images that may be seen on Apiject packaging or related materials.
Symbol
Standard and Reference #
Standard Title
FDA Recognition #
Date of Entry
Title
Description
EN ISO 15223-1:2021 Reference #5.1.1
5-134
12/20/2021
Manufacturer
Indicates the medical device manufacturer.
ISO 7000:2019 Reference #3082
5-124
12/23/2019
Manufacturer
Indicates the medical device manufacturer.

EN ISO 15223-1:2021 # 5.1.
5-134
12/20/2021
Authorized European representative
Indicates the Authorized representative in the European Community/European Union.

EN ISO 15223-1:2021 Reference # 5.7.10
5-134
12/20/2021
Unique Device Identifier
Indicates a carrier that contains unique device identifier information.
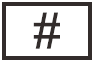
ISO 15223-1, Clause 5.1.10
5-134
12/20/2021
Model Number
Indicates the model number or type number of a product.
EN ISO 15223-1:2021 Reference #5.1.6
5-134
12/20/2021
Catalogue Number
Indicates the manufacturer‘s catalogue number so that the medical device can be identified

EN ISO 15223-1:2021 Reference #5.7.7
5-134
12/20/2021
Medical Device
Indicates the item is a medical device
EN ISO 15223-1:2021 Reference #5.1.5
5-134
12/20/2021
Batch Code
Indicates the manufacturer‘s batch code so that the batch or lot can be identified
ISO 7000:2019 Reference # 2492
5-124
12/23/2019
Batch Code
Indicates the manufacturer‘s batch code so that the batch or lot can be identified

EN ISO 15223-1:2021 Reference # 5.1.7
5-134
12/20/2021
Serial Number
Indicates the manufacture‘s serial number so a specific medical device can be identified.

ISO 7000:2019 Reference # 2498
5-124
12/23/2019
Serial Number
Indicates the manufacture‘s serial number so a specific medical device can be identified.

21 CFR 801.15(c)(1)(i)F
Labeling-Prescription devices
Labeling-Medical devices; prominence of required label statements
N/A
Prescription only
Requires prescription in the United States

21 CFR 801.109
Labeling-Prescription devices
Labeling-Prescription devices
N/A
Prescription only
Requires prescription in the United States
EN ISO 15223-1:2021 Reference #5.1.3
5-134
12/20/2021
Date of manufacture
Indicates the date when the medical device was manufactured
ISO 7000:2019 Reference # 2497
5-124
12/23/2019
Date of manufacture
Indicates the date when the medical device was manufactured
EN ISO 15223-1:2021 Reference #5.1.4
5-134
12/20/2021
Use-by date
Indicates the date after which the medical device is not to be used
ISO 7000:2019 Reference # 2607
5-124
12/23/2019
Use-by date
Indicates the date after which the medical device is not to be used

EN ISO 15223-1:2021 Reference #5.2.8
5-134
12/20/2021
Do not use if package is damaged and consult instructions for use
Indicates that a medical device that should not be used if the package has been damaged or opened and that the user should consult instructions for use for
additional information
additional information

ISO 7000:2019 Reference # 2606
5-124
12/23/2019
Do not use if package is damaged and consult instructions for use
Indicates that a medical device that should not be used if the package has been damaged or opened and that the user should consult instructions for use for
additional information
additional information

EN ISO 15223-1:2021 Reference #5.2.6
5-134
12/20/2021
Do not resterilize
Indicates a medical device that is not to be resterlized

ISO 7000:2019 Reference # 2608
5-124
12/23/2019
Do not resterilize
Indicates a medical device that is not to be resterlized
EN ISO 15223-1:2021 Reference #5.4.2
5-134
12/20/2021
Do not re-use
Indicates a medical device that is intended for one single use only
ISO 7000:2019 Reference # 1051
5-124
12/23/2019
Do not re-use
Indicates a medical device that is intended for one single use only

ISO 7000:2019
Reference #3079
Graphical symbols for use on equipment
Graphical symbols for use on equipment
5-124
12/23/2019
Open here
To identify the location where the package can be opened and to indicate the method of opening it.
EN ISO 15223-1:2021 Reference #5.4.3
5-134
12/20/2021
Consult instructions for use or consult electronic instructions for use
Indicates the need for the user to consult the instructions for use
ISO 7000:2019 Reference # 1641
5-124
12/23/2019
Consult instructions for use or consult electronic instructions for use
Indicates the need for the user to consult the instructions for use
EN ISO 15223-1:2021 Reference #5.2.3
5-134
12/20/2021
Sterilized using ethylene oxide
Indicates a medical device that has been sterilized using ethylene oxide
ISO 7000:2019 Reference # 2501
5-124
12/23/2019
Sterilized using ethylene oxide
Indicates a medical device that has been sterilized using ethylene oxide
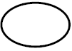
EN ISO 15223-1:2021 Reference #5.2.11
5-134
12/20/2021
Single sterile barrier system
Indicates a sterile barrier system

ISO 7000:2019 Reference # 3707
5-124
12/23/2019
Single sterile barrier system
Indicates a sterile barrier system

EN ISO 15223-1:2021 Reference #5.6.4
5-134
12/20/2021
Drops per Milliliter
Indicates the number of drops per milliliter

ISO 7000:2019 Reference # 2726
5-124
12/23/2019
Drops per Milliliter
Indicates the number of drops per milliliter

EN ISO 15223-1:2021 Reference #5.4.7
5-134
12/20/2021
Contains a medicinal substance
Indicates a medical device that contains or incorporates a medicinal substance.

ISO 7000:2019 Reference # 3702
5-124
12/23/2019
Contains a medicinal substance
Indicates a medical device that contains or incorporates a medicinal substance.

EN ISO 15223-1:2021 Reference #5.4.4
5-134
12/20/2021
Caution
Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or that the current situation needs operator
awareness
or operator action in
order to avoi descriptiond undesirable consequences
awareness
or operator action in
order to avoi descriptiond undesirable consequences

ISO 7000:2019 Reference # 0434A
5-124
12/23/2019
Caution
Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or that the current situation needs operator
awareness
or operator action in
order to avoi descriptiond undesirable consequences
awareness
or operator action in
order to avoi descriptiond undesirable consequences

MDR 2017/745, Article 20
REGULATION (EU) 2017/745 MDR
N/A
N/A
CE marking, If products are certified by an NB, the NB number is placed next to or below the CE mark symbol.
Signifies European technical conformity

EN ISO 15223-1:2021 Reference #5.7.8
5-134
12/20/2021
Translation
Indicates that the original medical device information has undergone a translation which supplements or replaces the original information

ISO 7000:2019 Reference # 3728
5-124
12/23/2019
Translation
Indicates that the original medical device information has undergone a translation which supplements or replaces the original information

EN ISO 15223-1:2021 Reference #5.7.4
5-134
12/20/2021
Patient information website
Indicates a website where a patient can obtain additional information on the medical product

ISO 7000:2019 Reference # 3705
5-124
12/23/2019
Patient information website
Indicates a website where a patient can obtain additional information on the medical product

EN ISO 15223-1:2021 Reference #5.3.1
5-134
12/20/2021
Fragile, handle with care
Indicates a medical device that can be broken or damaged if not handled carefully

ISO 7000:2019 Reference # 0621
5-124
12/23/2019
Fragile, handle with care
Indicates a medical device that can be broken or damaged if not handled carefully

EN ISO 15223-1:2021 Reference #5.3.2
5-134
12/20/2021
Keep away from sunlight
Indicates a medical device that needs protection from light sources

ISO 7000:2019 Reference # 0624
5-124
12/23/2019
Keep away from sunlight
Indicates a medical device that needs protection from light sources

EN ISO 15223-1:2021 Reference #5.3.4
5-134
12/20/2021
Keep dry
Indicates a medical device that needs to be protected from moisture

ISO 7000:2019 Reference # 0626
5-124
12/23/2019
Keep dry
Indicates a medical device that needs to be protected from moisture

EN ISO 15223-1:2021 Reference #5.3.7
5-134
12/20/2021
Temperature limit
Indicates the temperature limits to which the medical device can be safely exposed

ISO 7000:2019 Reference # 0632
5-124
12/23/2019
Temperature limit
Indicates the temperature limits to which the medical device can be safely exposed

EN ISO 15223-1:2021 Reference #5.3.8
5-134
12/20/2021
Humidity limitation
Indicates the range of humidity to which the medical device can be safely exposed

ISO 7000:2019 Reference # 2620
5-124
12/23/2019
Humidity limitation
Indicates the range of humidity to which the medical device can be safely exposed
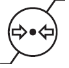
EN ISO 15223-1:2021 Reference #5.3.9
5-134
12/20/2021
Atmospheric pressure limitation
Indicates the range of atmospheric pressure to which the medical device can be safely exposed

ISO 7000:2019 Reference # 2621
5-124
12/23/2019
Atmospheric pressure limitation
Indicates the range of atmospheric pressure to which the medical device can be safely exposed
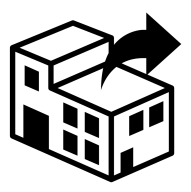
EN ISO 15223-1:2021 Reference #5.1.9
5-134
12/20/2021
Distributor
Indicates the entity distributing the medical device into the locale

ISO 7000:2019 Reference # 3724
5-124
12/23/2019
Distributor
Indicates the entity distributing the medical device into the locale

EN ISO 15223-1:2021 Reference #5.1.11
5-134
12/20/2021
Country of manufacture
To identify the country of manufacture of products
Additional Notes
Please send any questions to [email protected].